닫기
- In-Silico Method
- 인실리코 방법
Newly developed test methods in lieu of animal testing
Evaluate potential harmful ingredients based on chemical structure and similar substances which is not known in current market yet.
Based on prediction results, the potential harmful ingredients will be prohibited or limited use.
Introducing ChemTunes ToxGPS, a Chemoinformatics platform of MN-AM, an in-silico specialized organization in Germany, ensures data accuracy and reliability of prediction results through collaboration

QSAR
-
Predict potential for human toxicity/skin irritation induction based on chemical structure
Derivation of prediction results based on highly reliable regulation/toxicity DB of overseas regulatory agencies such as US FDA and EFSA [1]
US FDA Adopted Chemical Substance Risk Assessment Method [2]
Used in safety assessments at many global cosmetics companies
-
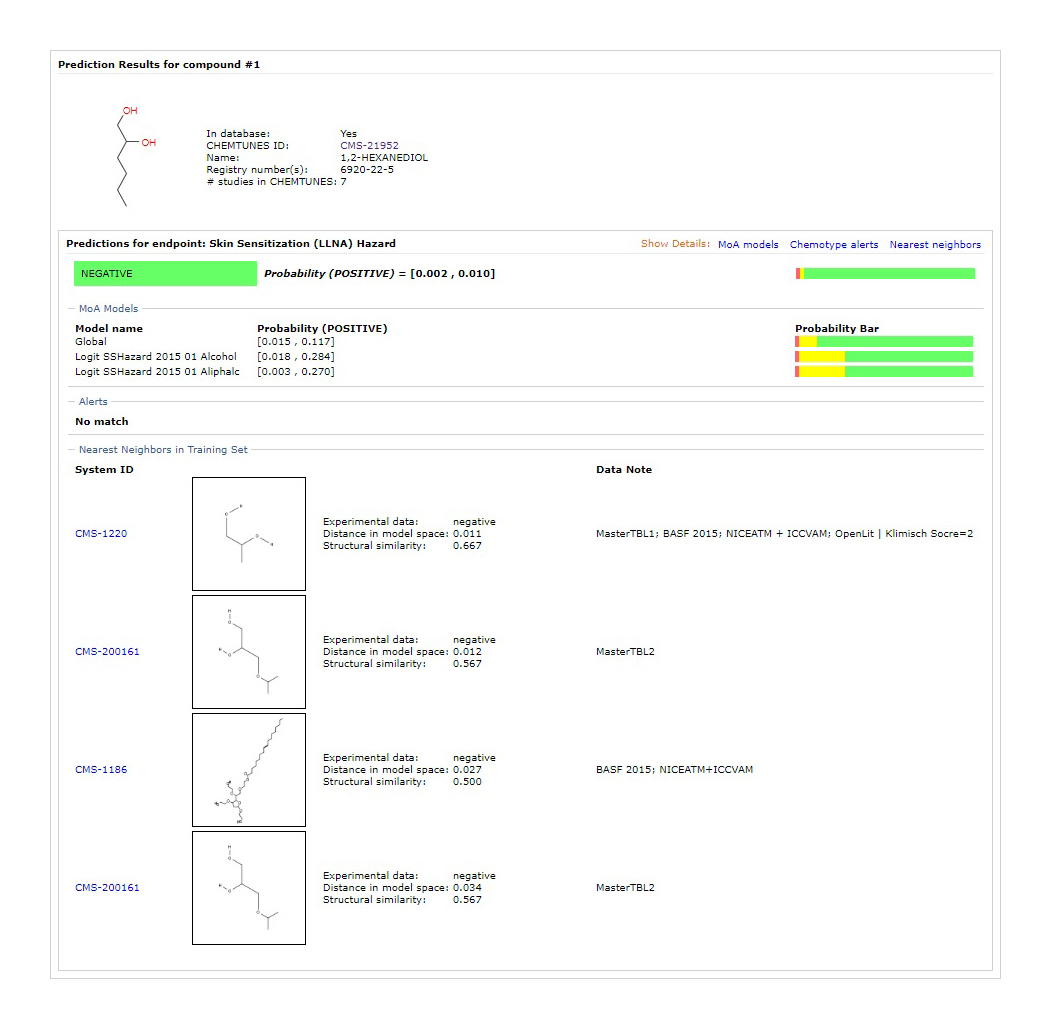
- Source - Company : MN-AM / Products : ChemTunes•ToxGPS
READ ACROSS
-
Predict the possibility of toxic/skin irritation of new components through correlation with substances whose toxicity information is known by grouping substances with similar functional groups, biological mechanism of action, or physicochemical patterns [3]
Derivation of prediction results based on highly reliable regulation/toxicity DB of overseas regulatory agencies such as US FDA and EFSA [1]
Secure raw material safety by grasping availability of new ingredients and available content based on predicted results
-
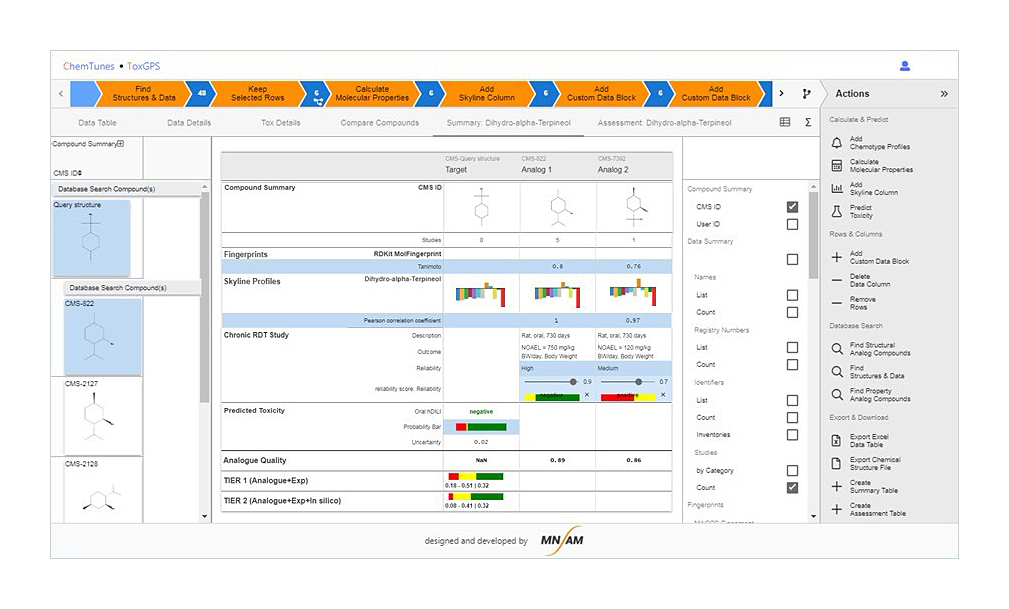
- Source - Company : MN-AM / Products : ChemTunes•ToxGPS
-
REFERENCE
[1] MN-AM (mn-am.com)
[2] Yang C, et al. Computational Toxicology Approaches at the US Food and Drug Administration. ATLA 2009, 37, 523-531.
[3] REACH Annex XI - Section 1.5 “Grouping of substances and read-across approach”
Reject E-mail collecting
The Cosmax website prevents and does not allow any act or attempt to collect e-mail addresses present in this website by programs for collecting e-mail addresses or by other technical means without our prior approval. Be advised that any violation of this provision may result in criminal penalties in accordance with the Information Network Act.


